Specialist Pharmaceutical Printers
Established 1986.
Specialist Pharmaceutical
Printers
Established 1986.
Specialist Pharmaceutical
Printers Established 1986.
Pharmaprint was initiated in 1986 and designed to offer quick turnaround, low cost, quality packaging with full traceability for the pharma/cosmetic industries.
We print REWIND (roll to roll), mainly aluminium foil for blister packaging and sachets and any packaging requiring a clean room type facility with full traceability during production.
The range of presses available can print orders ranging from a few kg to several tonnes. Narrow web machines allow us to print very small runs efficiently and with minimal waste (in most cases the roll is only handled once).
For larger orders multiple machines can be used and with again minimal waste.
Pharmaprint is able to produce very small runs for development trials, advertising promotions or just general small run contract packaging at both a competitive price and with very short lead times.
Our clean room facilities and S.O.P systems are approved by the major Pharma companies and are regulated by audits. This has helped make Pharmaprint a clear leader in their field. Pharmaprint is in the enviable position to be one of a few printers approved to print primary packaging (contact with product).
Pharmaprint was initiated in 1986 and designed to offer quick turnaround, low cost, quality packaging with full traceability for the pharma/cosmetic industries.
We print REWIND (roll to roll), mainly aluminium foil for blister packaging and sachets and any packaging requiring a clean room type facility with full traceability during production.
The range of presses available can print orders ranging from a few kg to several tonnes. Narrow web machines allow us to print very small runs efficiently and with minimal waste (in most cases the roll is only handled once).
For larger orders multiple machines can be used and with again minimal waste.
Pharmaprint is able to produce very small runs for development trials, advertising promotions or just general small run contract packaging at both a competitive price and with very short lead times.
Our clean room facilities and S.O.P systems are approved by the major Pharma companies and are regulated by audits. This has helped make Pharmaprint a clear leader in their field. Pharmaprint is in the enviable position to be one of a few printers approved to print primary packaging (contact with product).

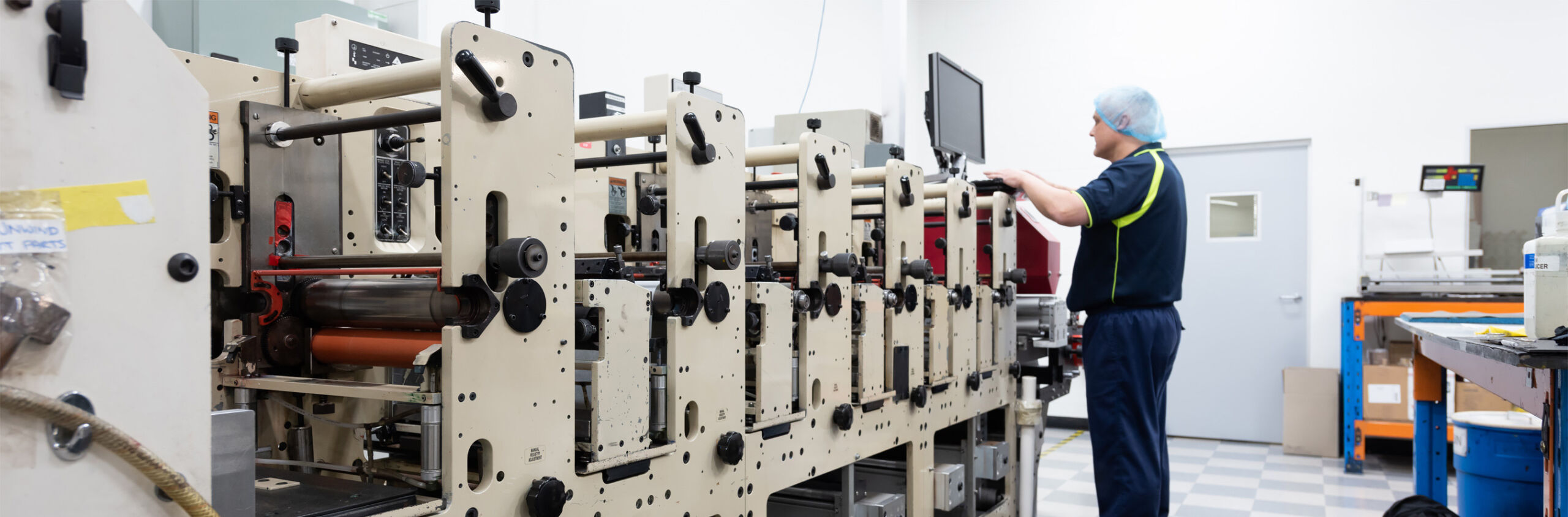
Facilities & Equipment
Pharmaprint’s machinery is based on narrow web inline presses with custom designed unwind and rewind systems to handle the intricacies of foil printing.
All our narrow web printing machines were designed and built in house specifically for these industries using our own engineers and design prompts from our clients.
A clean room facility is paramount to meeting the stringent requirements of the Pharmaceutical and food industries. Pharmaprint facilities are designed to be spacious, easy to keep clean and to allow an efficient workflow. Air supply is controlled, filtered and maintained at a positive pressure in accordance with GMP guidelines.
Pharmaprint’s machinery is based on narrow web inline presses with custom designed unwind and rewind systems to handle the intricacies of foil printing.
All our narrow web printing machines were designed and built in house specifically for these industries using our own engineers and design prompts from our clients.
A clean room facility is paramount to meeting the stringent requirements of the Pharmaceutical and food industries. Pharmaprint facilities are designed to be spacious, easy to keep clean and to allow an efficient workflow. Air supply is controlled, filtered and maintained at a positive pressure in accordance with GMP guidelines.
Facilities & Equipment

Pharmaprint’s machinery is based on narrow web inline presses with custom designed unwind and rewind systems to handle the intricacies of foil printing.
All our narrow web printing machines were designed and built in house specifically for these industries using our own engineers and design prompts from our clients.
A clean room facility is paramount to meeting the stringent requirements of the Pharmaceutical and food industries. Pharmaprint facilities are designed to be spacious, easy to keep clean and to allow an efficient workflow. Air supply is controlled, filtered and maintained at a positive pressure in accordance with GMP guidelines.

A clean room facility is paramount to meeting
the stringent requirements of the Pharmaceutical
and food industries. Pharmaprint facilities are
designed to be spacious, easy to keep clean and to
allow an efficient workflow. Air supply is controlled,
filtered and maintained at a positive pressure in
accordance with GMP guidelines.
A clean room facility is paramount to meeting
the stringent requirements of the Pharmaceutical
and food industries. Pharmaprint facilities are designed to be spacious, easy to keep clean and to allow an efficient workflow. Air supply is controlled, filtered and maintained at a positive pressure in accordance with GMP guidelines.
A clean room facility is paramount to meeting
the stringent requirements of the Pharmaceutical and food industries. Pharmaprint facilities are designed to be spacious, easy to keep clean and to allow an efficient workflow. Air supply is controlled, filtered and maintained at a positive pressure in accordance with GMP guidelines.


Our People
Our People
One of Pharmaprint strength is in its highly trained and motivated workforce.
All employees are trained using a robust and well-documented process with emphasis on attention to detail and the importance of quality. The stability of our workforce is evidenced by the low rate of staff turnover. Most of our employees have worked for Pharmaprint for more than 10 years.
One of Pharmaprint strength is in its highly trained and motivated workforce.
All employees are trained using a robust and well-documented process with emphasis on attention to detail and the importance of quality. The stability of our workforce is evidenced by the low rate of staff turnover.
Most of our employees have worked for Pharmaprint for more than 10 years.
One of Pharmaprint strength is in its highly trained and motivated workforce.
All employees are trained using a robust and well-documented process with emphasis on attention to detail and the importance of quality. The stability of our workforce is evidenced by the low rate of staff turnover. Most of our employees have worked for Pharmaprint for more than 10 years.


What we do
Pharmaprint operates it’s own prepress department capable of taking artwork from any format to proof ready for customer approval.
Prepress workflow is integrated with our approved plate supplier with prepress preparing files for plate output and completing quality control prior to issue.
Pharmaprint operates it’s own prepress department capable of taking artwork from any format to proof ready for customer approval. Prepress workflow is integrated with our approved plate supplier with prepress preparing files for plate output and completing quality control prior to issue.

What we do
Pharmaprint operates it’s own prepress department capable of taking artwork from any format to proof ready for customer approval.
Prepress workflow is integrated with our approved plate supplier with prepress preparing files for plate output and completing quality control prior to issue.

Quality System
Strong operating systems are required for efficient operation. A system of Standard Operating Procedures have been developed over the years with major input from our clients and following GMP guidelines to meet all our customers’ requirements.
The SOP system covers all areas of operation.
The use of SOPs is fundamental to the training program.
In support of the quality system a customised database is designed to produce required documentation for use by printers, to track all workflow & allow analysis of efficiency.
Quality System
Strong operating systems are required for efficient operation. A system of Standard Operating Procedures have been developed over the years with major input from our clients and following GMP guidelines to meet all our customers’ requirements.
The SOP system covers all areas of operation.
The use of SOPs is fundamental to the training program.
In support of the quality system a customised database is designed to produce required documentation for use by printers, to track all workflow & allow analysis of efficiency.
Local 02 9651 2777
Mobile 0417 164 382
International
011 +61 2 9651 2777
7am to 4pm
Monday to Thursday
Local 02 9651 2777
Mobile 0417 164 382
International
011 +61 2 9651 2777
7am to 4pm
Monday to Thursday
© Copyright Pharmaprint 2022
© Copyright Pharmaprint 2022


